COVID-19 creates new urgency for access to HIV self-testing, says global health agency Unitaid
The number of countries with self-testing policies in place has increased by nearly 15-fold since 2015, with an estimated 192 million self-tests needed to cover low- and middle-income countries by 2025.
Geneva – Disruptions and delays to HIV services caused by the COVID-19 pandemic led to declines in HIV testing and diagnoses in 2020 for the first time in more than two decades. HIV self-testing, which has contributed to a 40% reduction in the number of people who do not know their HIV status since 2015, is now crucial to maintaining access to HIV care for millions of people in the face of new COVID-19 roadblocks.
With more than 37 million people living with HIV worldwide – an estimated 6.1 million of whom do not know their status – receiving a diagnosis is a vital first step in accessing treatment.
Unitaid has led a large-scale effort to create access to HIV treatment and prevention through self-testing, with more than US$100 million invested since 2015. This work has helped reduce prices for self-tests, generate demand, demonstrate implementation pathways, and support scale up across 14 countries in Africa[1], with more recent work in India and Indonesia looking to further expand successful self-testing strategies to more people.
Data collected jointly by Unitaid and the World Health Organization show that between 2015 and 2020, the number of countries with self-testing policies has grown from six to 88 – and an additional 31 countries have policies in development. Eastern and Southern Africa, the epicentre of the HIV epidemic, are now leading the way with 52% of all countries implementing HIV self-testing – more than any other region. In contrast, significant gaps remain in Asia and Latin America, where just 10% and 6% of countries are implementing HIV self-testing respectively.
HIV self-testing has helped reach populations including men and young people who are less likely to access health centres and had significantly lower testing rates. In the largest evaluation of self-testing to date, Unitaid’s flagship STAR Initiative, led by Population Services International (PSI), found that, in Malawi, Zambia and Zimbabwe, close to half of all self-testers were men and more than one-third were people between the ages of 16 and 24.
Key populations who may hesitate to visit health centres due to stigma or discrimination, including men who have sex with men, transgender individuals, and sex workers, have also benefitted from self-testing. In West Africa[2], the Unitaid-funded ATLAS project targeted these groups by making self-tests available in places they frequent, such as nightclubs, hotels, or via personal contacts, with more than 40% of all people reached getting tested for the first time.
While procurement of HIV self-tests is rising, it is greatly outpaced by the need. By 2025, the number of self-tests procured is projected to fill just 15% of the estimated 192 million tests needed in low- and middle-income countries. And although the funding allocated for self-testing has increased significantly over the past several years, an additional US$104 million is needed to cover the costs of the 29 million self-tests expected to be procured.
Ahead of World AIDS Day, 1 December, Unitaid is calling for urgent funding and scale-up of HIV self-testing to protect progress threatened by COVID-19 and secure pathways to HIV treatment and prevention services for millions of people worldwide.
Disruptions and delays to HIV services caused by the #COVID19 pandemic led to declines in HIV testing and diagnoses in 2020 for the first time in more than two decades.
Read @UNITAID‘s statement for #WorldAIDSDay to find out more: https://t.co/COtIyXJVYI
— Unitaid (@UNITAID) December 1, 2021
[1] Unitaid funding has supported 14 countries in Africa with HIV self-testing; 11 through the STAR Project (Malawi, Zambia, Zimbabwe, eSwatini, Lesotho, South Africa, Cameroon, Mozambique, Nigeria, Tanzania and Uganda) and 3 countries through the ATLAS Project (Senegal, Mali, Cote d’Ivoire).
[2] Senegal, Mali, and Côte d’Ivoire
Media contact
For more information and media requests:
Maggie Zander
Communications officer
Mobile: +41 79 593 17 74
Email: zanderm@unitaid.who.int
Landscape for HIV rapid diagnostic tests for HIV self-testing – 2020
New paediatric formulation for HIV treatment hits the ground in six African countries
New paediatric formulation for HIV treatment hits the ground in six African countries
Child starting pediatric DTG 10mg at a health facility in Malawi. Credit: Lighthouse Trust.
Geneva, 4 October 2021 – On World AIDS Day 2020, Unitaid and the Clinton Health Access Initiative (CHAI) announced a groundbreaking deal that would see the very best HIV treatment made available to the youngest children for the first time.
Access for the youngest children
1.8 million children around the world live with HIV, the majority in low- and middle-income countries. Only 53% of these children are diagnosed and on treatment, while 80,000 babies and toddlers die each year from AIDS.
Ensuring access to treatments specifically designed for children is a key priority for Unitaid – and the agreement announced on December 1st 2020 saw the price for pediatric HIV treatment reduced by 75% with a new 10mg scored, dispersible formulation of dolutegravir.
Unitaid Executive Director Dr Philippe Duneton said: “To see this new paediatric formulation of DTG hitting the ground in six initial countries – and knowing that so many more are to come – is a huge moment for all the partners involved, and the communities that will benefit. Making the very best treatments available to the youngest children is at the heart of what Unitaid does and is vital if we are to achieve the global goals for HIV.”
It’s all in the taste
The strawberry-flavoured formulation of the WHO’s recommended first-line treatment for HIV has been designed to overcome many of the barriers that stop young children taking their medication properly.
A lack of appropriate paediatric medicines has meant that tablets are often unpalatable to children, due to a bitter taste or use of adult formulations being crushed or broken for children.
The collaboration between Unitaid, CHAI, originator ViiV Healthcare, and generic manufacturers Viatris and Macleods also led to the generic version of the medication receiving the fastest-ever United States FDA tentative regulatory approval.
“As a result of this unique collaboration alongside ministries of health and impacted communities, thousands of children in need will now receive the best HIV treatment available in the fastest time ever for a pediatric medication,” stated Joy Phumaphi, interim co-CEO of CHAI. “It is so exciting to see patients now accessing this lifesaving medication which has the potential to transform HIV care for children across low- and middle-income countries and save countless lives.”
Reaching those who need it most

DTG arrives in Zimbabwe for distribution. Credit: CHAI.
In May 2021, 100,000 packs of 10mg scored, dispersible DTG started hitting the ground in six African countries that were the target of a catalytic procurement by CHAI and Unitaid – Nigeria, Malawi, Uganda, Kenya, Zimbabwe and Benin.
Despite the difficulties of operating during the COVID-19 pandemic, CHAI – in close collaboration with Ministries of Health and national stakeholders – worked with procurement agent, i+ Solutions, to facilitate the purchase and delivery of the product in each of the six countries.
“The catalytic procurement by Unitaid and the support from CHAI has been a game changer, allowing faster introduction of this child friendly formulation to our young ones. This easy to administer formulation of pDTG is more efficacious, tastes better for the young palates, with more rapid viral load suppression and fewer side effects. The catalytic procurement is already being implemented in 13 high volume sites, and is expected to generate early experience, enabling younger children <20kgs access to the clinically superior benefits of DTG. Lessons learnt during first phase of implementation will inform national scale-up,” said Dr Angela Mushavi, National Prevention of Mother-to-Child Transmission and Pediatric HIV Care and Treatment Coordinator at the Ministry of Health and Child Care, Zimbabwe.
This initial procurement is designed to kickstart the demand in countries and help get the medication to as many of the 1.8 million children living with HIV who could benefit as possible.
Sister Mary Owens is the executive director of Nyumbani, an organization dedicated to providing HIV/AIDS services to children and affected families in Kenya since 1991.
Nyumbani children were among the first to receive the new DTG formulation, through Unitaid and CHAI’s catalytic procurement.
Sister Mary said: “Since the National AIDS Conference in Durban in 2016, I have been lobbying for access to DTG for our children. Since then, 102 of our children have been optimized as a result of our work alongside CHAI and Unitaid and the support of NASCOP to deliver these lifesaving medications. I am unable to express adequately our unbelievable joy that Nyumbani younger children can now access DTG 10mg – and are the first in Kenya and in Africa. We are already seeing the great benefit.”

Mothers of children living with HIV and key community leaders met at a workshop organized by the Community Advisory Board in Senegal. The meeting aimed to raise awareness about the availability and benefits of pediatric DTG and increase demand for the medicine. Credit: AfroCAB.
CHAI’s partnership with communities through the Optimal Community Advisory Board (CAB) is building demand and increasing patient treatment literacy on pediatric DTG 10mg.
Jacque Wambui, AfroCAB and CHAI-Unitaid Optimal CAB said: “It is important for communities and caregivers to be literate on paediatric DTG and how it is administered so as to maximize the benefits of using this formulation”.
The future
Major donors, including PEPFAR and the Global Fund, have rapidly moved to sustainable onward procurement, which will enable national scale-up and widespread access for all eligible children at an unprecedented pace.
The rollout of pDTG 10 mg dispersible, scored tablets is accelerating; more than 30 countries have plans for adoption and rapid rollout.
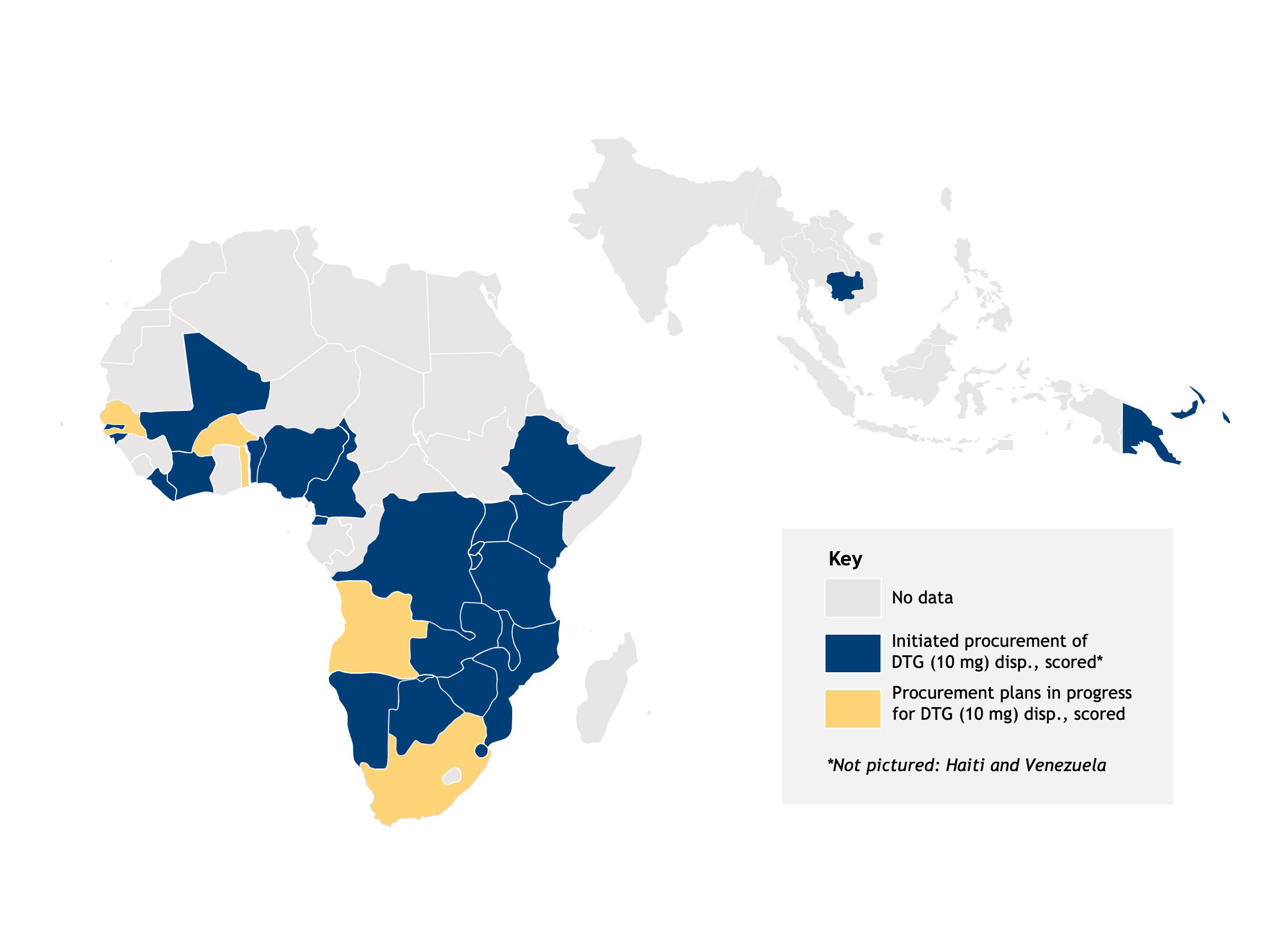
Map showing countries where adoption is planned.
Dr Angeli Achrekar, Acting U.S. Global AIDS Coordinator at PEPFAR, said: “Enabling sustainable access to paediatric DTG for all children living with HIV is a game changer and will greatly improve the health of children living with HIV worldwide.”
Read more about how paediatric DTG is transforming treatment for young children living with HIV.
About Unitaid
Unitaid is a global health agency engaged in finding innovative solutions to prevent, diagnose, and treat diseases more quickly, cheaply, and effectively, in low- and middle-income countries. Its work includes funding initiatives to address major diseases such as HIV/AIDS, malaria, and tuberculosis, as well as HIV co-infections and co-morbidities such as cervical cancer and hepatitis C, and cross-cutting areas, such as fever management. Unitaid is now applying its expertise to address challenges in advancing new therapies and diagnostics for the COVID-19 pandemic, serving as a key member of the Access to COVID-19 Tools (ACT) Accelerator. Unitaid is hosted by the World Health Organization.
Media contacts
For more information and media requests:
Hervé Verhoosel
Head of Communications
Unitaid, Geneva
tel. +44 77 29 618 634
Maggie Zander
Communication Officer
Unitaid, Geneva
tel. +41 79 593 17 74
Innovations in paediatric medicines delivery awarded UnitaidExplore funding
- Two new awards announced under Unitaid’s agility mechanism, UnitaidExplore; DelSiTech and FluidPharma will each receive investment for innovations to make medicines easier to give to children
- Latest call comes in context of Unitaid’s ground-breaking work on paediatric formulations to treat HIV, TB and malaria
- Children in low- and middle-income countries have lower treatment coverage and worse health outcomes than adults – a lack of paediatric formulations is a major contributing factor.
Geneva, 4 October 2021 – Two companies developing potentially game-changing medicine delivery mechanisms for children are the latest recipients of UnitaidExplore funding.
Finnish company DelSiTech and British enterprise FluidPharma have each been awarded funding following the latest call for applications under Unitaid’s pioneering agility mechanism.
Children in low- and middle-income countries have lower treatment coverage and worse health outcomes than adults. A major barrier is a lack of medication that is specifically formulated for their needs. Often medicine is too bitter, difficult to swallow or not correctly dosed, making it hard for children to stay on treatment for diseases such as HIV, malaria and TB.
The latest UnitaidExplore call specifically targeted this issue, inviting applicants to apply for funding to push forward innovation in this field. It builds on Unitaid’s significant work in the field of paediatric formulations for HIV, TB and malaria treatments, and its key role in WHO’s GAP-f network.
FluidPharma will use UnitaidExplore funding to take forward development of their MicroCoat™ technology, which utilises tiny cellulose spheres with taste-masking properties to deliver medication in a formulation that is more palatable to children. It is hoped that this technology could be used across a range of disease areas, with initial development of an artesunate/amodiaquine malaria combination therapy.
DelSiTech’s work focuses on the development of long-acting injectables to deliver medicines to children, reducing the burden of tablets and the associated stigma with taking such medication. The technology involves the use of thin, minimally invasive needles to deliver a unique silica-based formulation via sub-cutaneous injection. The technology can be used to administer drugs that treat or prevent a wide range of conditions, while significantly extending the effective duration of the treatment from a single dose.
Unitaid Director of Strategy Janet Ginnard said: “Innovations in medicine delivery that are specifically aimed at children are of utmost importance and we are pleased to announce this funding to DelSiTech and FluidPharma. These investments fit firmly with Unitaid’s track record in facilitating access to the best medicines for the most vulnerable people. These innovations will help ensure that children can benefit from lifesaving treatment and have the best possible health outcomes.”
The investments from Unitaid will accelerate both companies’ paediatric delivery mechanisms, covering pre-clinical work for several different potential applications.
Professor John Reeder from WHO’s GAP-f network said: “Unitaid’s new investments in innovative delivery approaches for children are extremely welcome, as they spark new energy and collaborations to ensure that science and innovation are at the service of those who have been too often left behind, our children.”
Dr. Lasse Leino, Chief Executive Officer from DelSiTech said: “Alliances, such as with Unitaid, are essential to us and to healthcare organisations around the world for the realisation of our common goal, securing real advancements in global health. DelSiTech is committed to pursuing long lasting strategic partnerships, enabling us to play a role in improving treatment outcomes, now for clearly underserved children. We are thrilled to collaborate with Unitaid and are prepared to leverage the full extent of our technologies and expertise for paediatric solutions for patients, wherever they may be”.
Dr Fang Liu from Fluid Pharma said: “We are really excited for this opportunity to join Unitaid’s excellent work in making medicines suitable for children. Applying the MicroCoatTM technology, we will develop paediatric anti-malarial treatments that are palatable, easy to swallow and stable, to improve compliance and treatment outcomes for children.”
This announcement complements two awards given last year to the first UnitaidExplore recipients, Vayu Global Health and EPFL EssentialTech, to take forward innovations in paediatric oxygen delivery.
About Unitaid
Unitaid is a global health agency engaged in finding innovative solutions to prevent, diagnose, and treat diseases more quickly, cheaply, and effectively, in low- and middle-income countries. Its work includes funding initiatives to address major diseases such as HIV/AIDS, malaria, and tuberculosis, as well as HIV co-infections and co-morbidities such as cervical cancer and hepatitis C, and cross-cutting areas, such as fever management. Unitaid is now applying its expertise to address challenges in advancing new therapies and diagnostics for the COVID-19 pandemic, serving as a key member of the Access to COVID-19 Tools (ACT) Accelerator. Unitaid is hosted by the World Health Organization.
Media contacts
For more information and media requests:
Hervé Verhoosel
Head of Communications
Unitaid, Geneva
tel. +44 77 29 618 634
Maggie Zander
Communication Officer
Unitaid, Geneva
tel. +41 79 593 17 74
Unitaid, the Clinton Health Access Initiative, and Laurus Labs announce agreement to accelerate development of best-in-class second- and third-line HIV medication for children
Geneva, 13 September 2021 – Development is underway based on an agreement launched in June 2021 between Unitaid, the Clinton Health Access Initiative (CHAI), and pharmaceutical manufacturer Laurus Labs to accelerate the development, commercialization, and registration of the best-in-class second- and third-line HIV treatment darunavir boosted with ritonavir (DRV/r) for children.
Despite being available in high-income countries for over a 15-year period, a generic, fixed-dose combination pediatric version of DRV/r is still not available. Through this agreement, Unitaid and CHAI are working with Laurus Labs to finally address this critical need and ensure CLHIV (Children Living with HIV/AIDS) have access to high-quality second- and third-line HIV treatment.
Unitaid Executive Director Dr. Philippe Duneton said, “Enabling children and young people to have access to the best possible HIV treatment is a key priority for Unitaid – whether that’s first, second, or third-line treatment. This agreement is a welcome step towards having a long-awaited pediatric formulation of DRV/r available, as we strive to achieve the global goal of 90% of people living with HIV receiving antiretroviral therapy.”
Second- and third-line therapies are critical for CLHIV where the World Health Organization (WHO)-recommended first-line treatment dolutegravir (DTG) may not be an option due to medication resistance or intolerance.
Currently available second- and third-line treatments are often difficult for children to take due to bitter taste and difficult forms of administration.
“A pediatric version of DRV/r has been a global health priority since 2013, but is still not available, leaving thousands of children without the lifesaving medication they need,” said Ann Veneman, interim Co-CEO of CHAI. “The innovative incentive program announced today will enable development of an effective pediatric medication that will save the lives of children living with HIV.”
The product development collaboration between Unitaid, CHAI, and Laurus Labs was spearheaded through Unitaid’s investment in CHAI since 2016 to bring the best HIV medications to market more quickly and integrate them into treatment programs in low- and middle-income countries that need them the most.
Understanding the small market size for a pediatric version of DRV/r, the initiative has provided Laurus with a financial incentive for a portion of their development and commercialization costs. CHAI will work closely with Laurus Labs to provide technical and regulatory support to enable accelerated generic development and regulatory submission of the medication.
Commenting on the collaboration in development of the new second line and third line pediatric treatment, Dr. Satyanarayana Chava, Founder & CEO, Laurus Labs, said, “we are excited about this innovative mechanism to accelerate the availability of pDRV/r regimen for children living with HIV.
The collaboration between Laurus Labs, CHAI, and Unitaid can positively impact over 100,000 CLHIVs and help them in leading a better life especially in their formative years.”
Background on DRV/r
DRV/r is a best-in-class protease inhibitor that has been shown to effectively treat HIV in children who are just beginning treatment, or whose treatment is no longer working. The WHO-led Pediatric Antiretroviral Drug Optimization (PADO) group, which establishes and reviews priorities for drug development for pediatric HIV treatment and prevention, lists pediatric DRV/r 120/20mg as a priority formulation to provide robust treatment for second- and third-line use after failure on DTG-based regimens. Despite being listed as a PADO priority since 2013, a pediatric version of DRV/r has not yet been developed.
About Unitaid
Unitaid is a global health agency engaged in finding innovative solutions to prevent, diagnose, and treat diseases more quickly, cheaply, and effectively, in low- and middle-income countries. Its work includes funding initiatives to address major diseases such as HIV/AIDS, malaria, and tuberculosis, as well as HIV co-infections and co-morbidities such as cervical cancer and hepatitis C, and cross-cutting areas, such as fever management. Unitaid is now applying its expertise to address challenges in advancing new therapies and diagnostics for the COVID-19 pandemic, serving as a key member of the Access to COVID-19 Tools (ACT) Accelerator. Unitaid is hosted by the World Health Organization.
About CHAI
The Clinton Health Access Initiative, Inc. (CHAI) is a global health organization committed to saving lives and reducing the burden of disease in low-and middle-income countries. CHAI works with its partners to help strengthen the capabilities of governments and the private sector to create and sustain high-quality health systems that can succeed without our assistance.
About Laurus
Laurus Labs is a fully integrated pharmaceutical and biotechnology company, with a leadership position in generic Active Pharmaceutical Ingredients (APIs) with a major focus on Anti-Retro Viral, Hepatitis-C, Cardiovascular, Anti-Diabetic, Central Nervous System (CNS), Proton Pump Inhibitors (PPIs) and Oncology drugs. Laurus Labs also develops and manufactures oral solid formulations, provides contract research and manufacturing services (CRAMS) to Global pharma companies, and produces specialty ingredients for nutraceuticals, dietary supplements and cosmeceuticals. Laurus Labs works on animal origin-free recombinant proteins in biotechnology.
Media contacts
For more information and media requests:
Clinton Health Access Initiative
Regan Lachapelle, Chicago, tel. +1 857-208-2788, rlachapelle@clintonhealthaccess.org
Unitaid
Maggie Zander, Geneva, tel. +41 79 593 17 74, zanderm@unitaid.who.int
Laurus Labs
Pavan Kumar N, Hyderabad, India, tel. – +91 7337353848, pavankumar.n@lauruslabs.com
Innovative agreement launches affordable, optimal second-line HIV treatment in low- and middle-income countries
- Unitaid and the Clinton Health Access Initiative, Inc. (CHAI) announce pricing agreement with pharmaceutical company Hetero Labs LTD to make available generic version of best-in-class second-line therapy for people living with HIV
Geneva, 26 July 2021 – Unitaid and CHAI today announced a groundbreaking pricing agreement with pharmaceutical company Hetero Labs LTD to make darunavir boosted with ritonavir (DRV/r) available as a second-line therapy for people living with HIV in low- and middle-income countries for US$210 per patient, per year.
Darunavir (DRV) in combination with ritonavir is a best-in-class protease inhibitor, proven to be effective both in patients who have never been treated for HIV and those who have experienced multi-drug resistance. DRV is easier to take and less toxic than existing protease inhibitors used in second-line treatment.
Despite being available in the United States and other high-income countries for over a decade, low- and middle-income countries still lack access to an affordable, quality generic version of the drug. Through this agreement, Hetero Labs LTD’s World Health Organization (WHO) prequalified DRV/r will be available in low- and middle-income countries for US$210 per patient per year, or US$17.50/pack (plus shipping and insurance). This price is cheaper than the well-established but suboptimal alternative option, lopinavir/ritonavir (LPV/r).
Kenly Sikwese, HIV advocate and head of the African Community Advisory Board (AfroCAB) stated, “It is fantastic to finally have a robust more efficacious protease inhibitor for the treatment of HIV. Since 2011, the community has been advocating for the use of DRV in second-line, and this milestone is a critical step to addressing the longstanding inequity in access to this optimal HIV treatment in low- and middle-income countries. We look forward to seeing policymakers across the globe move expeditiously forward to accelerate access to this very important drug for communities.”
Second- and third-line therapies are critical for people living with HIV where first-line treatment may not be an option, due to medication resistance or intolerance. However, there are barriers to commercializing DRV/r in low-resource settings, including the higher cost of producing the DRV active pharmaceutical ingredient in comparison to that of other protease inhibitors, as well as availability of DRV in a fixed-dose combination with ritonavir. Until now, this made the product too expensive for national treatment programs in low- and middle-income countries to afford, relying instead on sub-optimal products like LPV/r.
To address these barriers and others like them, Unitaid has invested in CHAI since 2016 to bring the best HIV medications to market more quickly and integrate them into treatment programs in the low- and middle-income countries that need them most.
Unitaid Executive Director Dr Philippe Duneton said, “Ensuring equitable access to the best HIV treatments is at the heart of what Unitaid does. This agreement with Hetero marks an important moment in accelerating the development and availability of a long-awaited generic formulation of darunavir/ritonavir. It is vital that all those living with HIV who cannot stay on first-line treatment have access to a quality second-line product, and we are proud of our work with CHAI to make this happen.”
Joy Phumaphi, CHAI Interim Co-CEO, stated, “Access to this best-in-class second-line HIV treatment is long overdue. We are grateful that through our partnership with Unitaid, patients in low- and middle-income countries will now be able to access the same high-quality second- line medication as those in high-income countries, enabling more patients to remain on treatment and save lives. We look forward to working with our government and community partners to quickly deliver this medication to patients.”
The agreement not only ensures a groundbreaking price, but that the product will be registered widely in low- and middle-income countries using the WHO Collaborative Registration Procedure (CRP) for prequalified products. The CRP accelerates registration through information sharing between the WHO Prequalification of Medicines Programme (PQP) and national medicines regulatory authorities.
To reach the milestone announced today, CHAI and Unitaid drew on years of experience on both the supply and demand side of the HIV therapy market. CHAI engaged with the originator company, Janssen, and generic drug manufacturers to encourage data sharing, and ensure a generic product was quickly developed and filed with stringent regulatory and national drug regulatory authorities. Unitaid provided an innovative financial incentive to secure a highly competitive yet sustainable price, that is designed to partially offset the high costs of manufacturing until economies of scale are achieved. These efforts helped ensure that affordable pricing was available in low- and middle-income countries from day one, thereby paving the way for adoption of the product.
About Unitaid
Unitaid is a global health agency engaged in finding innovative solutions to prevent, diagnose, and treat diseases more quickly, cheaply, and effectively, in low- and middle-income countries. Its work includes funding initiatives to address major diseases such as HIV/AIDS, malaria, and tuberculosis, as well as HIV co-infections and co-morbidities such as cervical cancer and hepatitis C, and cross-cutting areas, such as fever management. Unitaid is now applying its expertise to address challenges in advancing new therapies and diagnostics for the COVID-19 pandemic, serving as a key member of the Access to COVID-19 Tools (ACT) Accelerator. Unitaid is hosted by the World Health Organization.
About the Clinton Health Access Initiative, Inc. (CHAI)
The Clinton Health Access Initiative, Inc. (CHAI) is a global health organization committed to saving lives and reducing the burden of disease in low-and middle-income countries. We work with our partners to strengthen the capabilities of governments and the private sector to create and sustain high-quality health systems that can succeed without our assistance. For more information, please visit: www.clintonhealthaccess.org.
Media contacts
Maggie Zander, Unitaid, Geneva | zanderm@unitaid.who.int | tel. +41 79 593 17 74
Regan Lachapelle, Clinton Health Access Initiative, Chicago | rlachapelle@clintonhealthaccess.org | tel. +1 857-208-2788
