End-of-Project Evaluation of the HIV Self-Testing AfRica (STAR) Initiative
Access to HIV self-tests significantly expanded and costs halved thanks to Unitaid agreement
- Self-testing vital tool to help people know HIV status – but market has been dominated by single oral-based self-test
- New Unitaid-led agreement with Viatris (through its subsidiary Mylan) reduces price of blood-based HIV self-test by 50%, significantly expanding market and giving countries more options
- New test will be available for less than $2 in 135 countries
- Self tests key factor in achieving SDG target of 90% of people infected with HIV knowing their status
Geneva – Self-testing is a vital tool to help people discover their HIV status, giving individuals a pathway to start treatment and reducing the HIV burden globally. This is particularly relevant in low- and middle-income countries (LMICs), where concerns around stigma and difficulties in accessing healthcare can put up significant barriers.
The market for HIV self-tests in LMICs has improved since Unitaid first invested in 2015, but has been dominated by a single affordable test – with other later options costing at least $1 more.
Today, Unitaid is announcing a significant market expansion and price reduction of around 50%, thanks to an agreement with Viatris (through its subsidiary Mylan), which will see blood-based HIV self-tests made available for less than $2 each in 135 eligible countries.
The agreement follows a request for proposals launched by Unitaid and Population Services International (PSI) in 2020 to drive forward equitable access to these tests.
Access to self-tests has been recognised as a key factor in meeting the global goal of 90% of people knowing their HIV status. In the past six years, this rate has already increased from 45% to 81%.
Unitaid Director of Programmes Robert Matiru said: “HIV self-testing is a crucial factor in helping people learn their status – it is one of the key ways in which the global goals for HIV will be achieved. This announcement today will have a concrete impact on the ability of countries to access affordable self-testing, a foundation of people-centred healthcare in which Unitaid has led the way.”
This market expansion will give countries more choice when it comes to self-tests, making it easier to acquire the products and embed them in health systems, with the ultimate aim of helping the 8 million people estimated to be unaware of their HIV status know they are infected and get treatment.
Dr Meg Doherty, Director of Global HIV, Hepatitis and STI Programmes at WHO said: “WHO welcomes the wider availability of affordable HIV self-testing kits to increase access to testing. This announcement is particularly timely now, as HIV self-testing has become an important choice during COVID-19, allowing people to test when other options are difficult to access or restricted.”
Dr Thato Chidarikire, Director of HIV Prevention Strategies at the National Department of Health of South Africa, said: “With over 2 years of implementing HIV self-screening in South Africa, we have seen the positive impact the intervention has had on the programme. We have managed to reach men, women between 19 and 24 years old, as well as key populations, which was the main aim of the intervention. The news of reduced prices for blood-based HIV SS test kits is very well-received by South Africa, as we are currently procuring the tests using domestic funding. Lower prices translate to more quantities and expansion of the programme to reach more untested and test-averse populations, contributing to the country reaching the 95-95-95 targets.”
The Mylan HIV self-test – which is manufactured by Atomo Diagnostics Limited – and another recently-developed blood-based HIV self-test from Abbott (currently undergoing regulatory review) will also form the basis of an access expansion programme from Unitaid, which will see around 1 million tests distributed to stimulate in-country demand.
This announcement builds on the success of Unitaid’s STAR and ATLAS projects, which have seen access to self-testing drastically expanded in Africa and Asia.
To date, Unitaid investment has resulted in 5 million kits being distributed, with 21 million kits set to be procured by countries between 2020 and 2023. Additionally, self-testing protocols have been embedded in the health policies of more than 85 national governments.
- Improving HIV prevention for mother and child. Hiv self-testing to prevent mother-to-child transmission. PSI, Photo Story
Background notes
The Request for Proposal (RfP) was launched 7 July 2020 by Population Services International and Unitaid, as part of the intervention to accelerate market access and scale up of blood-based HIV Self-Testing kits in resource-limited settings and priority countries. The bidder(s) were expected to provide a comprehensive proposal including an access price, not more than the target price of US$2 or lower, with associated terms and supply conditions.
The proposals were expected to include complementary contributions from the manufacturer to improve the delivery and scale-up of HIVST through public and private sector channels via innovative delivery and product demand generation strategies. The proposed intervention is aimed at ensuring a healthy market with enough quantities of tests to meet this demand at an affordable and sustainable price.
Media contacts:
Charlotte Baker | bakerc@unitaid.who.int | +447904 460 181
Hervé Verhoosel | verhooselh@unitaid.who.int | +44 77 29 618 634
Accelerating access to innovative point-of-care HIV diagnostics: Lessons learned from UNICEF
Unitaid funding sees launch of world’s first long-acting medicines centre at University of Liverpool
Geneva – Efforts to revolutionise treatments for debilitating infectious diseases have been amplified today with the launch of a new research centre at the University of Liverpool.
Established as part of a US$40 million international research consortium, primarily funded by Unitaid, the University of Liverpool’s Centre of Excellence for Long-acting Therapeutics (CELT) will be the first of its kind in the world.
By repurposing existing medicines into slow-release formulations, where drug effectiveness can be sustained over several months, ‘long-acting’ technology has already been successfully implemented in the fields of contraception and schizophrenia.
It now has the potential to improve the outcomes for treatment and prevention of deadly diseases such as HIV, malaria, Hepatitis C and tuberculosis, which particularly impact low- and middle-income countries.
Current treatment courses for these conditions have often resulted in poor outcomes in low-resource environments, as those living with diseases struggle with regimens that can involve taking dozens of tablets every day and rely on regular access to healthcare settings.
CELT’s mission is to broaden knowledge of long-acting medicines and disseminate key research, with the aim of revolutionising how these devastating diseases are treated, particularly in countries where access to healthcare is challenging.
The work will be conducted out of two state-of-the-art laboratories at the University of Liverpool, where the development of long-acting formulations for malaria and TB prevention, as well as a single-injection cure for hepatitis C, is already under way as part of the Unitaid-funded LONGEVITY project. In the case of malaria prevention, for example, the aim is to cover an individual for the entire malaria season with just one injection.
Meanwhile, by facilitating collaboration between scientists from the fields of pharmacology and materials chemistry, as well as global partners, CELT will ensure that the long-acting medicines are carefully designed with the specific needs of affected communities in mind.
Other projects focus on helping researchers understand better the key success factors for oral, injectable and implantable long-acting approaches.
Unitaid’s Executive Director Dr Philippe Duneton said: “Decades ago, long-acting products revolutionised fields such as schizophrenia and contraception. Today, our goal is to apply similar innovation to bolster global efforts to tackle – and even eliminate – major diseases affecting low- and middle-income countries, including HIV/AIDS. The pipeline of new long-acting products is promising. As a funder of catalytic health interventions, we are excited and inspired to be supporting the University of Liverpool, and other partners, that are blazing a trail in that regard.”
Co-director of CELT, Professor Andrew Owen, said: “Long-acting drug delivery promises to transform patient management, with huge potential impact for treatment and prevention of infectious diseases. Benefits for efficacy flow from overcoming issues associated with patients sometimes not taking their medication, which may also help reduce emergence of antimicrobial resistance. CELT harnesses the power of local, national and international collaboration to accelerate understanding of the medicines of the future.”
World’s first long-acting medicines centre launches today @LivUni, thanks to Unitaid funding. CELT’s aim is to broaden knowledge of innovative formulations to make an impact in #TB, #malaria, #HepC and other diseases. https://t.co/lgzaALjWcq pic.twitter.com/B3k5D6PP19
— Unitaid (@UNITAID) January 12, 2021
Media contact: Charlotte Baker | +44 7904 460 181 | bakerc@unitaid.who.int
Five things about paediatric DTG
Unitaid reaffirms its commitment to the fight against HIV in the context of the COVID-19 pandemic
This World AIDS Day 2020, Unitaid reaffirms its commitment to the fight against HIV, while calling for innovative solutions to overcome stagnating progress towards global targets and challenges presented by the COVID-19 pandemic.
Despite significant gains achieved in the fight against HIV, global targets for 2020 have been missed.
- In 2019, there were 1.7 million new infections, with key populations accounting for 62% of new infections globally, and young women and girls accounting for 48% in sub-Saharan Africa.
- Of the 38 million people living with HIV in 2019, 12 million individuals did not have access to treatment.
Global efforts to meet international targets were already off-track in 2019, and progress has been further derailed by the COVID-19 pandemic.
Since April 2020, in 36 countries which are home to almost half of all people on antiretroviral therapy (ART), disruptions in the provision of HIV services have been reported. It has been widely acknowledged that the interruption of HIV services could lead to an increase in HIV mortality and incidence during the pandemic, with estimates by the WHO and UNAIDS suggesting that COVID-19 related disruptions could lead to up to 293,000 new infections and up to 148,000 additional deaths to HIV globally through 2020.
Within the context of this new pandemic, it is more critical than ever to ensure that both people living with HIV and those most at-risk for acquiring HIV remain a priority and have access to uninterrupted HIV services. A lack of access to these services puts a person living with HIV at increased risk of treatment disruption, contracting co-infections and Advanced HIV disease.
HIV and related co-infections make up the largest segment of Unitaid’s $1.3 billion grant portfolio. Our grantees report that nearly all grants have been impacted by COVID-19 to varying degrees. These impacts have been mitigated with innovative tools and approaches to maintain service continuity and ensure that program’s impact is not compromised. The disruption to HIV programmes has been mitigated by three areas of innovation:
- Accelerated access to simplified tools and people-centred approaches
- Wider use of digital technologies
- Optimising outreach and community involvement.
“Our efforts over the last several months have been to ensure continuity of services for HIV, and to leverage as much as possible knowledge gained by the HIV programmes to address COVID-19 and its consequences over other public health areas”, said Unitaid Executive Director Philippe Duneton. “Innovations have been rapidly deployed to address these colliding pandemics, simultaneously achieving progress against both objectives and enabling better approaches to improve our efforts in the future. Our commitment towards all populations living with HIV – adults, adolescents and children – is paramount in the context of this double pandemic.”
Read more from October’s Hummingbird about our partners’ work in this area.
Making optimised health technologies and tools ready for scale-up in low and middle-income countries will bring us closer to achieving the new global targets for ending HIV as a public health threat.
Looking ahead, Unitaid will continue to work towards identification and introduction of innovative solutions to simplify and render more effective prevention, testing and treatment of HIV, co-infections and comorbidities, with a focus on people-centred approaches that place individuals living with HIV, or at high risk of infection, and their communities, at the centre of the HIV response.
Related:
- Groundbreaking Agreement Reduces by 75% the Cost of HIV Treatment for Children in Low-and Middle-Income Countries, Unitaid, 1.12.2020
- Five things about paediatric DTG
- Unitaid-funded HIV/AIDS projects (Website)
- Unitaid and HIV/AIDS (Factsheet)
- Unitaid HIV portfolio (Infographic)
Media contact:
- Charlotte Baker, Unitaid, Geneva | tel. +44 7904 460 181 | bakerc@unitaid.who.int
Groundbreaking Agreement Reduces by 75% the Cost of HIV Treatment for Children in Low-and Middle-Income Countries
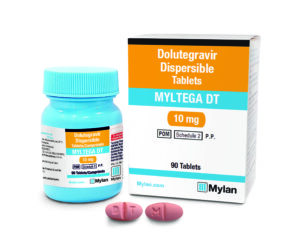
New formulation is dispersible and strawberry-flavoured, enabling the youngest children living with HIV to be treated with the best available medication
New price agreement with Viatris and Macleods will significantly lower cost for yearly paediatric HIV treatment from over $480 per child to under $120 per child[1]
Innovative partnership has accelerated development of first generic paediatric dispersible formulation of dolutegravir (DTG), the recommended first-line HIV treatment
A long-awaited HIV treatment designed specifically for children will now be available in low-and middle-income countries, thanks to a landmark agreement from Unitaid and the Clinton Health Access Initiative (CHAI).
1.7 million children around the world live with HIV, but only half of them receive any treatment and 100,000 die every year. For many of these children, the HIV virus is not suppressed, due in part to the lack of availability of effective drugs that are palatable and properly adapted for them.
The new pricing agreement with generic manufacturers Viatris and Macleods means a new dispersible formulation of the recommended first-line HIV treatment dolutegravir (DTG) will be launched at a yearly cost of $36 per child, reduced from around $400[2].
The innovative partnership between Unitaid, CHAI and ViiV Healthcare[3], together with Mylan (now a subsidiary of Viatris), has resulted in the fastest ever regulatory approval under the US FDA PEPFAR programme of a generic paediatric HIV drug.
“Children in low- and middle-income countries often wait years to access the same medications as adults, hindering their quality of life, or even resulting in preventable deaths,” said Unitaid’s Executive Director Philippe Duneton. “This groundbreaking agreement will bring quality assured dispersible DTG to children at a record pace. Ensuring access to this treatment will transform the lives of children living with HIV, helping them to remain on treatment and saving thousands of lives.”
Many children living with HIV have a poor response to treatment because they take anti-retroviral medication that is not correctly dosed or bitter to taste. Despite being the World Health Organisation-recommended first-line treatment for children since 2018, an affordable DTG has been unavailable to children under 20kg so far, due to a lack of dispersible tablets which are considered age-appropriate formulations.
“It’s time to fight back against substandard HIV treatment outcomes in children,” said Dr Meg Doherty, Director of Global HIV, Hepatitis and STI Programmes at WHO. “Today we can finally guarantee that countries have rapid access to the appropriate formulations needed to fully implement WHO guidelines; so that no child is left behind. WHO welcomes the approval and commercialisation of the new pediatric DTG 10 mg. Congratulations to all the partners involved for showing how quickly we can bring new formulations to market when we work together – clear proof that solidarity delivers results.”
This new DTG 10 mg strawberry-flavoured, dispersible tablet is more inviting to children. It will enable them to successfully remain on medication and prevent thousands of premature deaths each year, transforming paediatric HIV treatment in low and middle-income countries.
The agreement will also significantly lower the total annual cost of paediatric HIV treatment from $480 per child to less than $120. With global health budgets more constrained than ever, such significant savings – in the range of US$60-260 million over five years – will be a game-changer.
This announcement coincides with the U.S. Food and Drug Administration’s (FDA) tentative approval of the first generic paediatric dispersible DTG product from Viatris on November 19th 2020. This is the first time a generic product has been positively reviewed within several months of the originator product receiving FDA approval, reducing the gap from three years for the adult version of the same medicine, to just five months. This filing strategy represents a major innovation which could drastically reduce the time it takes for new paediatric medications to reach children in low- and middle-income countries. Tentative approval for Macleods’ product is expected in early 2021.
CHAI CEO Iain Barton said: “This innovative collaboration will, for the first-time, enable children living with HIV in low-and middle-income countries to access the same first-line ARV medication at the same time as those in high-income countries. The partnership should serve as a model to remove barriers that hinder development of paediatric formulations to deliver top-line medications quickly and affordably.”
Unitaid, CHAI, and national Ministries of Health are partnering with the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) to drive early access to the product in several countries to generate feedback on early use, to help inform wider adoption and scale-up.
“PEPFAR is deeply committed to optimizing HIV treatment for individuals of all ages,” said Ambassador Deborah L. Birx, U.S. Global AIDS Coordinator and U.S. Special Representative for Global Health Diplomacy. “The availability of pediatric DTG formulations offers a significant opportunity for young children living with HIV to access robust, child-friendly treatment that will directly improve their health. PEPFAR will continue to collaborate with global and local partners to support the accelerated introduction and widespread use of pediatric DTG among the children we serve.”
The product will be made initially available in Benin, Kenya, Malawi, Nigeria, Uganda and Zimbabwe in the first half of 2021, with plans for rapid scale-up of dispersible DTG 10 mg across a broad set of countries.
Cabinet Secretary for the Ministry of Health in Kenya, the Hon. Mûtahi Kagwe, said: “This announcement marks a dramatic shift for the quality of HIV treatment for children. Kenya intends to be a first-adopter of the new paediatric DTG 10mg formulation, which will improve treatment, reduce unpleasant side effects, and help children to adhere to their treatment and live healthy lives. We are delighted that for the first time Kenya and other countries can provide children the same quality of treatment as adults, which has been made possible through the development of this new formulation.”
Related:
- Unitaid reaffirms its commitment to the fight against HIV in the context of the COVID-19 pandemic, Unitaid, 1.12.2020
- Five things about paediatric DTG
- Unitaid-funded HIV/AIDS projects (Website)
- Unitaid and HIV/AIDS (Factsheet)
- Unitaid HIV portfolio (Infographic)
[1] $480 per year based on average price of currently available, recommended first line therapy using ABC/3TC dispersible tablets and LPV/r pellets or granules for a child from 10.0-13.9 kg; $120 per year based on currently available ABC/3TC dispersible tablets and new DTG 10 mg scored dispersible tablet for a child from 10.0-13.9 kg (based on WHO weight bands). Current product prices per USAID GHSC-PSM September 2020 Product e-Catalog.
[2] $404 is the average annual cost of LPV/r pellets and granules for a child from 10.0-13.9 kg. Current product prices per USAID GHSC-PSM September 2020 Product e-Catalog.
[3] Viiv Healthcare provided a Technical Transfer package to support the development of this new formulation
Background notes
DTG has been recommended by the World Health Organization (WHO) as the preferred first-line treatment regimen for children over the age of four weeks and greater than 3 kg since 2018, but to date only children weighing 20 kg or more have been able to access the medication due to a lack of dispersible, age-appropriate formulations for younger children.
During the COVID-19 pandemic, it is more critical than ever to ensure that all people living with HIV achieve and maintain viral suppression. An unsuppressed viral load puts a person living with HIV at increased risk of contracting co-infections, such as tuberculosis.
The product development collaboration between Unitaid, CHAI, and ViiV Healthcare was spearheaded through Unitaid’s investment in CHAI since 2016 to bring the best HIV medications to market more quickly and integrate them into treatment programs in communities in developing countries that need them the most. The initiative provided Viatris and Macleods with a financial incentive for development and registration. ViiV Healthcare, the originator of DTG, shared technical knowledge and expertise and CHAI provided technical and regulatory support that enabled accelerated generic development and regulatory submission. ViiV Healthcare received FDA approval of their paediatric dispersible 5mg dolutegravir tablet in June 2020.
The new generic formulations of DTG manufactured by Viatris and Macleods will be available at the lower price to 121 countries covered by ViiV Healthcare’s pediatric DTG voluntary licensing agreements via the Medicines Patent Pool, covering 99% of children living with HIV. Through supporting better treatment, and with recent advances in earlier diagnosis of infants born with HIV, we finally have the tools to achieve the goal of ending HIV-related child mortality.
Media contacts:
- Charlotte Baker, Unitaid, Geneva | tel. +44 7904 460 181 | bakerc@unitaid.who.int
- Regan Lachapelle, CHAI, Boston | tel. +1 857-208-2788 | rlachapelle@clintonhealthaccess.org

