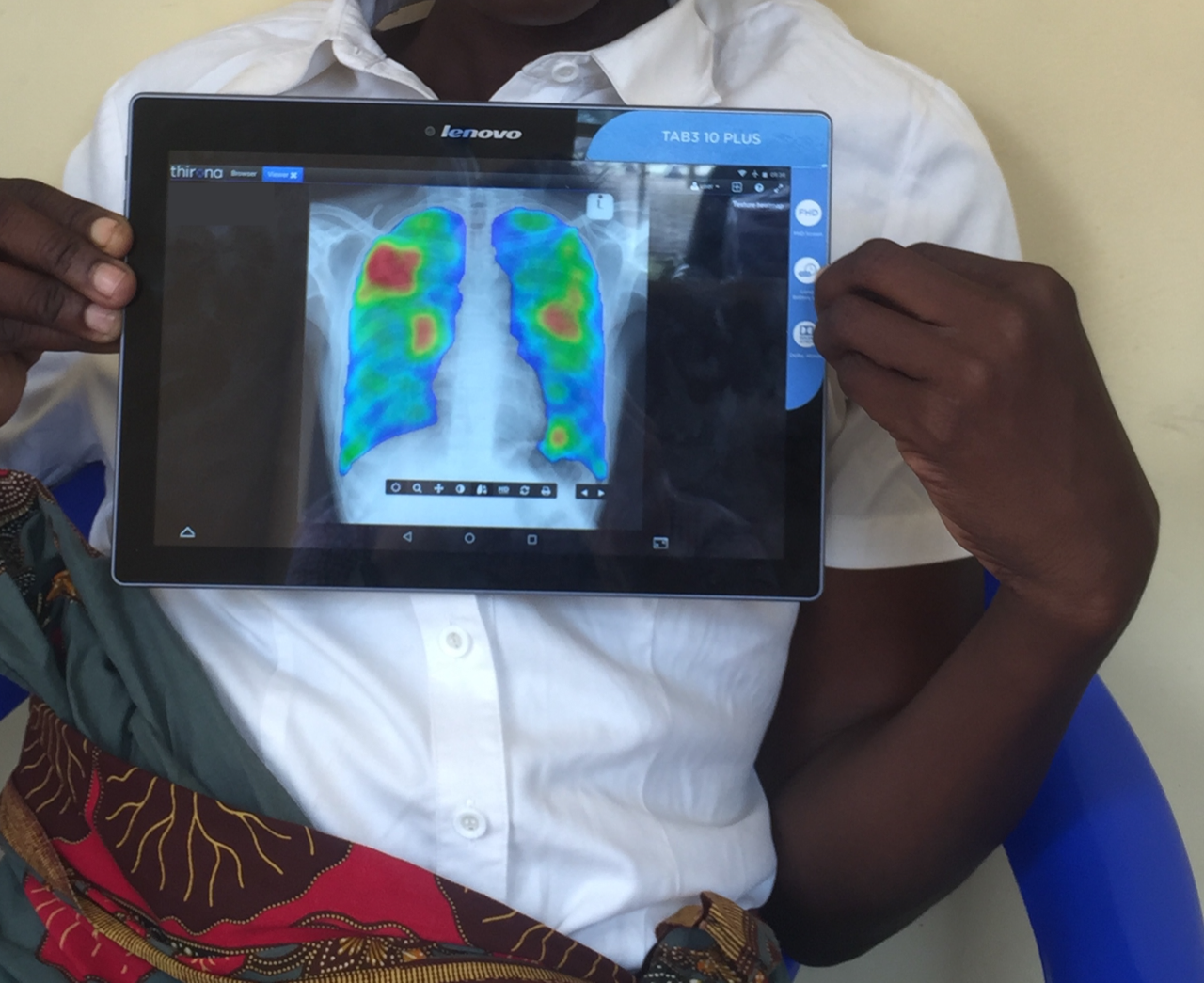
Expanded options with available drugs
Safe for vulnerable groups
More affordable option
MDR-TB occurs when TB bacteria is resistant to two or more of the first-line drugs used to treat it, making treatment far more complex. Like TB, MDR-TB is airborne. A person can catch MDR-TB from another infected individual or drug resistance can develop over time if the disease is not treated properly.
Less than a decade ago, the standard MDR-TB treatment lasted 18 to 24 months and required taking tens of thousands of pills. Injectable drugs, used in many of the longer regimens, were painful and caused extreme side effects such as psychosis or permanent hearing loss. This made treatment arduous for patients, and greatly impacted treatment completion and success rates.
Newer, shorter, all-oral treatment regimens have cut treatment duration down to as little as six months for some patients. Additional treatment regimens, including three regimens developed through the Unitaid-backed endTB project, were recommended by the WHO in 2024 as the first short-course MDR-TB regimens approved for all populations, finally enabling children, adolescents, and pregnant and breastfeeding women to access shorter treatments.
Testing medicines in children or pregnant women requires careful attention to ethical, safety and practical considerations. Because these populations are more vulnerable, they are often excluded from clinical research, which can severely limit their access to critical medicines. Additionally, because children represent a smaller percentage of the population, companies may misjudge the value of investing in the research that would allow them to benefit from new medicines.
With more than US$80 million invested in the endTB project, we have made a significant contribution to advancing shorter, more effective, and less toxic treatment regimens for MDR-TB.
Observational research in 17 countries generated critical evidence on the safety and efficacy of two new drugs for treating MDR-TB, bedaquiline and delamanid. This work helped shape national treatment guidelines and informed the historic 2018 WHO recommendation to switch away from painful injections to less toxic, all-oral regimens, and the subsequent 2022 revision of WHO’s global MDR-TB treatment recommendations.
Meanwhile, the endTB project led a multi-country clinical trial to evaluate new treatment regimens for MDR-TB, which resulted in a WHO recommendation of three new treatments using medicines that are more affordable, already registered and available in low- and middle-income countries, and recommended for use in all populations.