Shorter treatment
Safe for all populations
Reduced prices
Cost effective
TB can be prevented by finding and treating people with active disease to stop transmission, and by proactively finding and treating people who have been exposed to TB with preventive medicines before they become symptomatic.
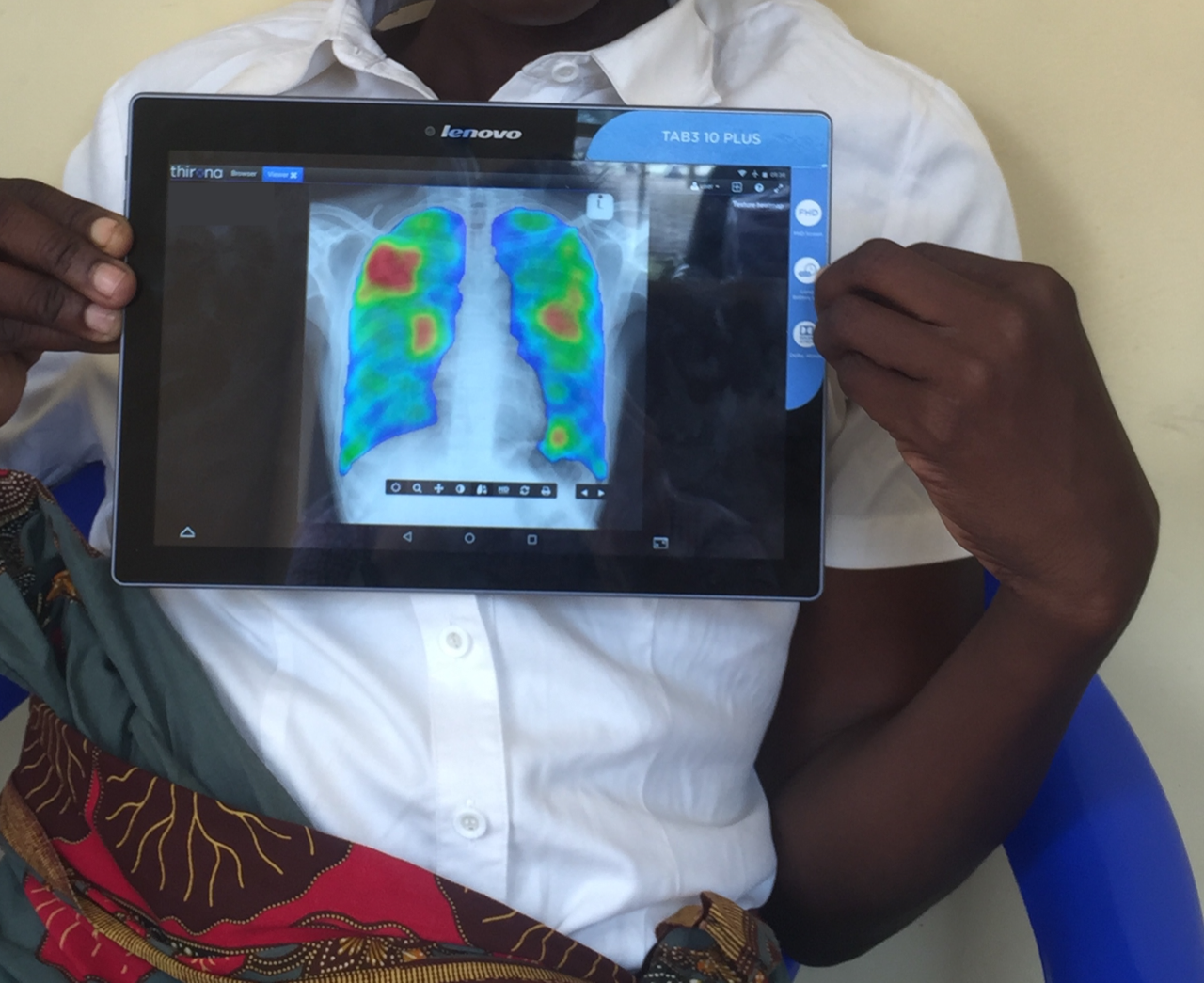
The TB bacteria can remain in a person’s body for weeks, months or even years without causing symptoms and without being contagious. This is called TB infection, and up to one-quarter of the world’s population may have it. TB infection can be treated with a course of antibiotics that kill the TB bacteria before it has a chance to make a person ill. Without TB preventive treatment, 5 to 10% of people with TB infection will develop TB disease. Young children and immuno-compromised individuals, like people living with HIV or another coinfection, are even more likely to develop active disease after exposure.
There are two short-course regimens. They both use a combination of the antibiotics isoniazid and rifapentine and require either once-weekly treatment over three months (3HP) or daily treatment over one month (1HP). These preventive regimens represent a dramatic improvement on previous treatments that lasted anywhere from six to 36 months. Because previous preventive therapies were so long and could cause side effects, treatment initiation and completion – and therefore treatment success – was limited. These shorter regimens now provide a simpler, more effective way to clear TB infection and prevent disease.
People who live with someone with TB disease are at significantly higher risk of progressing to active disease than the general population. Young children and others with fragile immune systems such as people living with HIV or other health conditions are at the highest risk.
TB is the leading cause of death among people living with HIV. High-quality evidence proving that 3HP is safe and effective when taken at the same time as the first-line HIV medicine dolutegravir has enabled millions of people with HIV to access critical preventive care, exceeding the target set at the first United Nations high-level meeting on TB to reach 6 million people with HIV by 2022.
An estimated 2.25 million children and adolescents should get TB preventive treatment each year due to their HIV status or exposure to TB in the home. With the newly developed pediatric formulation of rifapentine now available and affordable to use with existing isoniazid formulations, children now have access to child-friendly TB prevention medicines.
With our support, uptake of short-course TB prevention regimens has increased dramatically. Together with our partners, we brought the price of 3HP down by 80% and worked with manufacturers to increase supply, growing production from 180,000 patient courses in 2018 to 4.5 million a year in 2024. Our support for demand creation has seen country procurement of 3HP grow to 11 million patient courses in 101 countries in 2024, compared to 70,000 patient courses in a single country when our work began.
To ensure those most vulnerable to TB disease could access the best TB preventive therapies available, we funded clinical trials to evaluate their safety and efficacy when used in combination with leading HIV medicines, in children and pregnant women, and developed child-formulations to facilitate care.
Analysis published in The Lancet found that the lives of 850,000 people could be saved by 2035 if short-course TB preventive treatment is provided to people living with HIV and household contacts of those newly diagnosed with TB. 700,000 of those lives saved would be among children aged 15 years and younger.