Prevention and treatment
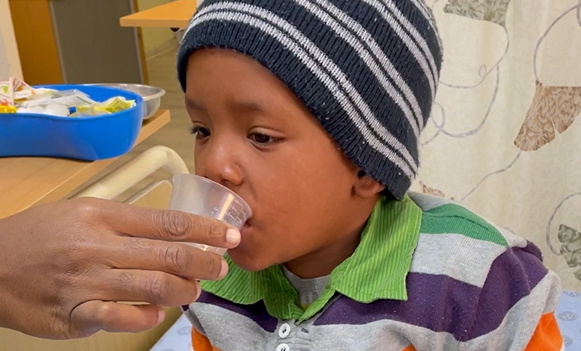
Fruit flavored
Properly dosed
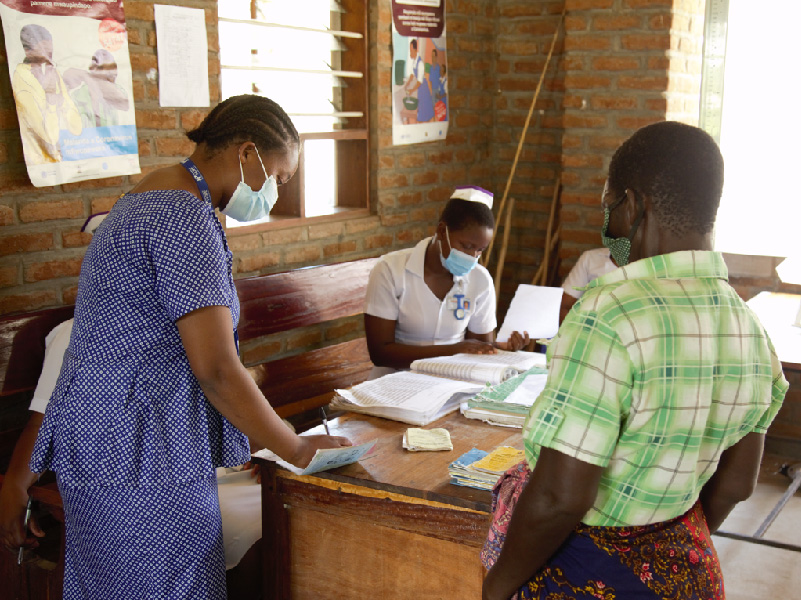
Prioritizes the most vulnerable
Pediatric formulations are versions of medicines that can be used to treat children. Producing them generally requires adapting adult medicines so they taste better and are easier to administer, particularly to small children who cannot swallow pills. Developing child-friendly medicines also typically includes investing in additional research to understand proper dosages for children of different ages and weights.
Because medicines tend to be developed with the broadest possible recipient community in mind, companies usually address adult needs first. Adaptations for children often come several years later, if they are developed at all.
Developing medicines for children is perceived to be costly, time-consuming, and requiring more complex ethical considerations, though it may not always be the reality. Because children may represent a smaller or more unpredictable market, companies sometimes lack the incentive to develop child medicine formulations. This is especially true for diseases such as TB that primarily affect people in low- and middle-income countries where the potential for profit does not appropriately reflect the critical need for child medicines.
This is where Unitaid’s role is crucial. We fund the development of affordable child formulations, to correct for the historic neglect of children in global health responses.
For decades, children suspected of having TB were treated with approximate doses of crushed-up bitter-tasting adult medicines. Caregivers struggled to get children to swallow the pills, and many children were unable to complete treatment, leading to countless preventable deaths.
Unitaid first invested in child TB medicine development in 2013, resulting in the world’s first high-quality, properly dosed TB drugs for kids. We have continued this work since, having now developed child-friendly formulations of all the medicines used in major TB and MDR-TB treatments, including the first nine-month MDR-TB regimens recommended for children by the World Health Organization (WHO).
Because children are highly susceptible to developing active TB disease after exposure to the infection, high-quality preventive care is equally vital. As part of our efforts to improve access to shorter, more effective TB preventive treatments, we supported the development of child-adapted rifapentine for prevention, which is now available at record low prices, in many cases less than the cost of adult regimens.
Further research evaluated the first-ever treatment for preventing MDR-TB in children. This evidence, which was presented at the same time as a similar study into adult MDR-TB prevention, informed the WHO’s recommendation. With our support, researchers simultaneously developed and registered a pediatric formulation of levofloxacin, the key medicine used in the MDR-TB preventive therapy, to enable implementation of the recommendation using the highest quality, most appropriate medicine formulations without delay.